Soldiers fire at targets during a live-fire training at Grafenwoehr Training Area, Germany, July 31, 2020.
Wednesday, August 05, 2020
DOD Awards $104 Million for Procurement of Syringes in Support of U.S. COVID-19 Vaccination Campaign
On August 4, the Department of Defense’s (DOD) Joint Acquisition Task Force (JATF), in support of the Department of Health and Human Services’ (HHS) Strategic National Stockpile (SNS), awarded $104 million in contracts to procure syringes and safety needles, enabling the nationwide administration of a U.S. Food and Drug Administration (FDA)-approved COVID-19 vaccine, once one is available. The syringes and safety needles are critical to the nation’s COVID-19 vaccination strategy, providing a total of 500 million safety syringes over a 12-month period, with more than 134 million of the total number delivered by the end of 2020.
The syringes and safety needles will be placed into the SNS so they will be readily available to quickly and efficiently vaccinate the U.S. population once a safe and efficacious COVID-19 vaccine is developed. This procurement is the latest in a series of recent contracts highlighting a collaborative “whole-of-government” approach in response to the COVID-19 threat.
The Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND) partnered with HHS and the Army Contracting Command – Aberdeen Proving Ground (ACC-APG), to select and award contracts to six companies: Duopross Meditech Corporation ($48 million), Cardinal Health Inc. ($15 million), Gold Coast Medical Supply, LP ($14 million), HTL STREFA Inc. ($12 million), Quality Impact, Inc. ($9 million), and Medline Industries, Inc. ($6 million). The companies represent a mix of large and small medical product manufacturers and distributors.
“This effort demonstrates skillful collaboration between the interagency and our industry partners, and moves the nation farther forward in its fight against COVID-19,” said Joint Program Executive Officer for Chemical, Biological, Radiological and Nuclear Defense, Douglas Bryce. “Immediate procurement and prepositioning of syringes will enable rapid vaccination of the U.S. population once an FDA-approved vaccine becomes available. We are pleased to be part of this unprecedented and critical undertaking.”
This CARES Act funded effort is part of the ongoing collaboration between DOD and HHS to support replenishment of the SNS with critical medical resources.
Esper Discusses COVID-19, Inclusion, National Defense Strategy
Defense Secretary Dr. Mark T. Esper discussed the National Defense Strategy and U.S. great-power competition, the challenges COVID-19 brought to the Defense Department and his initiatives on diversity and inclusion in a virtual address to the Aspen Security Conference.
"When I was confirmed over a year ago, … one of the things I made clear is my top priority would be implementing the National Defense Strategy," the secretary said in today's remarks. "As you know, the NDS says we are now in an era of great power competition, [and] our chief competitors are China and Russia, in that order."

A second tier of countries must be addressed, he added, such as North Korea and Iran, and the enduring threat of violent extremist organizations also figures into the equation.
Since Esper took office, DOD has been moving out for nearly 13 months
in terms of implementing the NDS and its three lines of effort:
One of the challenges DOD has faced over the previous seven months is the impact of COVID-19, which hit the department in January, the secretary said.
"I've been tracking this since mid-January, and the department received its first U.S. citizens from China in late January," he said. DOD put its global defense plan into effect Feb. 1.
"We've been at this for seven months, and I'm really proud of the Department of Defense," Esper said. "I think at our high point, there were over 60,000 service members on the streets of America, in many of the hot spots — particularly the National Guard — whether it was medical professionals helping out in hospitals to [those] distributing supplies."
"I'm very proud of what the United States military did — often putting themselves at risk," the secretary said.

And during that challenging time, the secretary outlined three priorities: to take care of DOD's people and their families, ensure the department could maintain our readiness to execute the national security mission and to support the whole-of-government effort.
On top of that, he noted, DOD dealt with civil unrest in the wake of the murder of George Floyd. "Our service members again did extremely well in serving their state governors … making sure that Americans had the right to exercise their First Amendment right of freedom of speech and assembling to protest, and to do that peacefully, so I've been very proud of our Guard in that manner," Esper said.
The civil unrest led the secretary to pursue three initiatives to address racial discrimination, diversity and inclusion in the military ranks.
"Clearly, while the U.S. military has been a leader in dealing with discrimination in its ranks and making sure it is not part and parcel of our force, we are not immune to what's happening in broader society, so we're taking a number of actions to do that for two reasons," the secretary said.
"First, it's the right thing to do. But secondly, it's important to our readiness," he said. "We need to be able to recruit the best and the brightest and to make sure they all feel respected, regarded and have all the opportunities that everybody else does in our force. And that applies not just to persons of color, but to ethnic differences, gender, sexual orientation, you name it. We want to represent the American people that we've sworn an oath to protect and defend."
DOD is focused inward and is helping inward and at the same time, maintaining its national security capabilities and defending the country, Esper said. "And we see that as our top priority," he added, "and [we] are committed to continue to do so as we move forward."
Okinawa Reception Center Welcomes Marines, Sailors Despite Pandemic
''It's a challenge,'' said Marine Corps Lance Cpl. Preston Batson, the assistant barracks manager for the Joint Reception Center Marines. New Marines are required to fulfill a 14-day restriction-of-movement order when they arrive on Okinawa. ''The JRC staff and I are always on the go,'' Batson said. ''While we wear masks and stay 6 feet apart, there's always a chance to be exposed to COVID-19. We are surrounded by Marines new to the island who have executed the 14-day restriction of movement and Marines whose flight has just landed to Okinawa.''

Despite COVID-19 restrictions, JRC Marines have continued to assist hundreds of accompanied and unaccompanied service members with their overseas transition.
New, unaccompanied Marines who are E-5 and below and ''greenside'' sailors — Navy personnel attached to a Marine Corps unit — step off the Patriot Express into the Kadena Air Base terminal to be greeted by the JRC staff. Batson, eager to welcome the Marines and sailors, helps facilitate the process of checking in the Marines and ensuring their well-being.
''Our job is continuous,'' said Batson, also a driver for JRC. ''We live with the JRC Marines, so when we go home we are still with them — always on call.''
Though all inbound personnel to Okinawa must comply with the 14-day restriction of movement, the JRC staff helps ensure their safety by conducting wellness checks three times a day, bringing meals to them and ensuring cleanliness.

''On average, we have 60 or more Marines and sailors in both barracks,'' said Batson. ''COVID-19 has not stopped JRC; we have only adjusted what we do.''
After the 14-day ROM cycle, the Marines execute their week-long orientation to the island. Before the pandemic, the JRC Marines had classes every day, and, toward the end of the week, they would visit Hacksaw Ridge. Health protection condition restrictions limit the staff to teaching only the most vital classes. Under current conditions, the battle site tours have been suspended.
''COVID-19 has put a hold on some of the things we do, but we always adapt and overcome,'' said Marine Corps Cpl. Daniel Chavez, a troop handler with JRC. ''It's kept us on our toes, and we adjusted, as needed, to continue helping the Marines in JRC.''
With COVID-19 still prevalent, it has caused an adjustment to the organization and procedures of JRC; however, the staff has worked hard to overcome and push forward.
''There is a lounge for the JRC Marines to interact with one another — of course, staying 6 feet apart and wearing a mask,'' Batson said. ''There is a TV, video games, board games and books for them to use. The biggest thing is to make sure they are OK. I don't like to see a Marine sad. I want to do everything I can to make them smile and know we are here for them.''

The JRC staff helps establish principles for other noncommissioned officers to uphold when placed in a leadership position with Marines under their command.
''In a way, we are a mentor to these Marines,'' said Chavez. ''NCOs should be watching out for their Marines, making sure they get chow, and be somebody who helps with anything they need.''
The main effort of the JRC staff is to take care of every Marine and sailor who comes through the program.
''At the end of the day, it's our job as the JRC staff to make sure the new Marines and sailors are safe,'' said Batson. ''A part of keeping them safe is checking on them multiple times a day and ensuring they are healthy, physically and mentally. They are important.''
(Marine Corps Lance Cpl. Karis Mattingly is assigned to Marine Corps Installations Pacific.)
Navy Reservists Aid Front-Line Medical Staff
"I'm just grateful to help during this time," said Navy Petty Officer 2nd Class Jon Doliana, a hospital corpsman. Doliana was deployed to the COVID-19 respiratory clinic activated at Great Lakes.
"I love being here doing whatever the Navy needs me to do," the Nashville, Tennessee, native said.
As COVID-19 became a global concern, the Lovell Health Care Center altered many of its practices to safeguard patients and staff, but the mission didn't change. Since it was established in 2010 as the nation's first fully integrated federal health care center in support of both the Defense and Veterans Affairs departments, one of the center's main missions is medically preparing Navy recruits for military service. The pandemic has not diminished the need for military readiness and it has greatly increased the amount of medical resources needed to accomplish that goal.
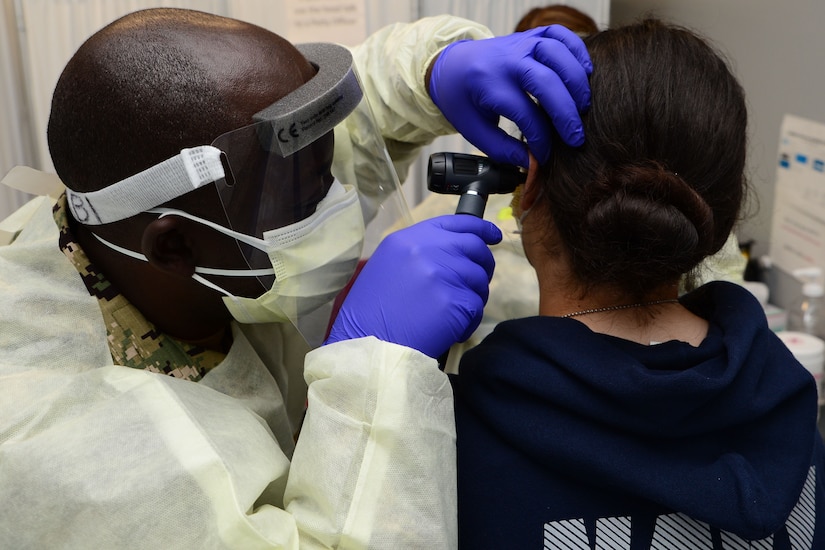
On June 3, a select team of 81 Navy Reserve medical personnel mobilized to Great Lakes as part of the DOD's response to the pandemic. Reservists were deployed from their hometowns and from multiple units — including the Expeditionary Medical Facility Bethesda, Maryland, and the Naval Reserve Center Nashville, Tennessee — to augment the fleet's active-component staff. The team is led by Navy Capt. Rebecca Zornado from Oceanside, California. Teams include hospital corpsmen and members of the Medical Service Corps, Medical Corps, Nurse Corps, and other enlisted support staff, including personnel support and yeomen.
As part of the strategy to prevent the spread of COVID-19 throughout the boot camp population, the Navy restricted the off-base movement of all Navy recruits at a nearby lodge and hotels before they began their boot camp, and the nursing staff checks the "pre-recruits" daily for COVID-19 symptoms. The screening includes an evaluation for both typical and atypical symptoms.
"It's been a huge benefit having the reservists onboard," said Navy Cmdr. (Dr.) Robert Senko, who usually is the director of primary care medicine at Lovell FHCC but has been overseeing the recruit restriction-of-movement process since it was implemented. Senko said the reservists have stepped up and taken charge of the respiratory clinics and sick call, as well as restriction-of-movement medical procedures.
The nurses assigned to the staff teams supervise the delivery of care and nasopharyngeal swabbing procedures to ensure optimal screening standards and appropriate use of personal protective equipment, which all medical staff in direct contact with recruits use. The nurse practitioners also are responsible for screening, diagnosing and treating recruits.

The extra manpower is necessary as Lovell FHCC allows more face-to-face appointments with veterans and more recruits enter the restricted-movement period.
In addition to the extra effort needed to control the spread of COVID-19, Lovell FHCC's usual recruit health and dental care mission continues. Annually, it involves about 40,000 recruits. The facility operates three RTC branch medical clinics. At USS Red Rover, recruits receive immunizations and other medical procedures before being declared fit for full duty; the USS Osborne provides a full spectrum of dental services for recruits. But the USS Tranquility is the busiest, providing services from that range from sick call to mental health care and special physicals. The reservists have lightened the load.
Each reserve team implemented standard operating procedures for health protection in their work environment, including the use of personal protective equipment.

When asked what the biggest challenge of being deployed to Great Lakes during the COVID-19 pandemic is, Navy Chief Petty Officer Lisa Tran, a hospitalman from Houston, is unequivocal.
"Keeping up with the current research, which may change the next day, and keeping our corpsmen trained on the best medicine, the best practice at the time is somewhat of a challenge," she said. "But it's nothing we can't handle."
(Navy Seaman Recruit Minh-Thy Chu is assigned to the Captain James A. Lovell Federal Health Care Center.)
Bougainville Blast
Marine Corps Pfc. Bart Bryant fires a tube-launched, optically-tracked, wireless-guided missile during at Pohakuloa Training Area, Hawaii, July 18, 2020, during Bougainville II, the second phase of a predeployment exercise designed to increase combat readiness through complex and realistic live-fire training.
HHS, DOD Collaborate With Johnson & Johnson to Produce Millions of COVID-19 Investigational Vaccine Doses
The U.S. Department of Health and Human Services (HHS) and Department of Defense (DoD) announced an agreement with the Janssen Pharmaceutical Companies of Johnson & Johnson, to demonstrate large-scale manufacturing and delivery of the company’s COVID-19 vaccine candidate. Under the terms of the agreement, the federal government will own the resulting 100 million doses of vaccine.
The vaccine doses could be used in clinical trials or, if the U.S. Food and Drug Administration (FDA) authorizes use as outlined in agency guidance, the doses would be distributed as part of a COVID-19 vaccination campaign.
“With the portfolio of vaccines being assembled for Operation Warp Speed, the Trump Administration is increasing the likelihood that the United States will have at least one safe, effective vaccine by 2021,” said HHS Secretary Alex Azar. “Today’s investment represents the next step in supporting Janssen’s vaccine candidate all the way through manufacturing, with the potential to bring hundreds of millions of safe and effective doses to the American people.”
This manufacturing demonstration project will take place while clinical trials are underway. Working in parallel this way expedites the traditional vaccine development timeline. This step builds toward the U.S. government’s Operation Warp Speed goal to begin delivering millions of doses of safe and effective vaccines to the American people by the end of the year.
The Biomedical Advanced Research and Development Authority (BARDA), part of the HHS Office of the Assistant Secretary for Preparedness and Response, collaborated with the DoD Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense and Army Contracting Command, to provide approximately $1 billion to support the manufacturing demonstration project including the ability to deliver vaccine doses to government-designated locations across the country. The government also can acquire additional doses up to a quantity sufficient to vaccinate 300 million people.
The project announced today includes fill-finish manufacturing in U.S.-based facilities. This fill-finish manufacturing step ensures vaccine doses are packaged and ready to ship immediately, subject to successful clinical trials and FDA authorization.
If these doses are used in a COVID-19 vaccination campaign, the vaccine would be available to the American people at no cost. As is customary with government-purchased vaccines, healthcare professionals could charge for the cost of administering the vaccine.
To date, BARDA has provided approximately $456 million for clinical trials and other vaccine development activities under an existing, long-term partnership with Janssen. The company’s investigational vaccine relies on Janssen’s recombinant adenovirus technology, AdVac, a technology used to develop and manufacture Janssen’s Ebola vaccine with BARDA support; that vaccine received European Commission approval and was used in the Democratic Republic of the Congo (DRC) and Rwanda during the 2018-2020 Ebola outbreak that began in the DRC.
About Operation Warp Speed
OWS is a partnership among components of the Department of Health and Human Services and the Department of Defense, engaging with private firms and other federal agencies, and coordinating among existing HHS-wide efforts to accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics.
About HHS, ASPR, and BARDA:
HHS works to enhance and protect the health and well-being of all Americans, providing for effective health and human services and fostering advances in medicine, public health, and social services. The mission of ASPR is to save lives and protect Americans from 21st century health security threats. Within ASPR, BARDA invests in the innovation, advanced research and development, acquisition, and manufacturing of medical countermeasures – vaccines, drugs, therapeutics, diagnostic tools, and non-pharmaceutical products needed to combat health security threats. To date, BARDA-supported products have achieved 55 FDA approvals, licensures or clearances. To learn more about federal support for the nationwide COVID-19 response, visit coronavirus.gov.
About the JPEO-CBRND:
The Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND) protects the Joint Force by providing medical countermeasures and defense equipment against chemical, biological, radiological and nuclear (CBRN) threats. JPEO-CBRND’s goal is to enable the Joint Force to fight and win unencumbered by a CBRN environment. JPEO-CBRND facilitates the rapid response, advanced development, manufacturing and acquisition of medical solutions, such as vaccines, therapeutics, and diagnostics, to combat CBRN and emerging threats such as COVID-19. To learn more about JPEO-CBRND’s COVID-19 response, visit https://www.jpeocbrnd.osd.mil/coronavirus or follow JPEO-CBRND on social media at @JPEOCBRND.









